| |
Is there an effect of positional plagiocephaly on neurodevelopmental delay in infants and toddlers?
By Mike Marinus MSc (Paeds) (M.Tech Chiro)
Mike Marinus MSc (Paeds), 91 Hornbill, Douglasdale, South Africa, 0832894037
Institution: AECC University College, Bournemouth England BH5 2DF
Contact: drmarinus@gmail.com
ABSTRACT
Objective: Since the Back to Sleep campaign in 1992, the incidence of positional plagiocephaly continues to increase substantially. A body of work is emerging linking positional plagiocephaly to neurodevelopmental delay, including data that reveals a physical shift in brain parenchyma in response to skull asymmetry. This review assesses the nature of the relationship between these neurodevelopmental delays and positional plagiocephaly. Method: A literature search was required to answer the clinical question. Pubmed, Medline and The Cochrane Library were searched using the mesh terms: ‘plagiocephaly, nonsynostotic’ and ‘growth and development’ in conjunction with the terms: ‘neurodevelopmental delay’, development’ and ‘delay’. After the relevant inclusion and exclusion criteria were applied, 12 studies were reviewed. Results: Positional plagiocephaly has shown a defined link to neurodevelopmental delay in infants. The effect is seen more prominently in motor skills during infancy and the delay has been noted to extend into preschool age children. Plagiocephaly patients are more likely to have altered muscle tone. No correlation was seen between the severity of the skull asymmetry and the level of neurodevelopmental delay experienced by the child. Conclusion: The data suggest correlation but not necessarily causation. It is also possible that pre-existing neurodevelopmental delay may be the cause of positional plagiocephaly. In most cases it is likely to be a combination of the risk factors of supine sleep, lack of prone awake time, variable muscle tone, low activity levels, male gender and neck muscle dysfunction that attributes to the delays that have been recognized in these infants.
Key words: Positional Plagiocephaly, neurodevelopmental delay, Chiropractic, Pediatric.
Introduction
Chiropractors are involved in the diagnosis and treatment of neuromusculoskeletal disorders affecting the pediatric population, and as such may note varying degrees of skull deformity in infants during the physical examination. There are anecdotal findings which suggest this is widespread and that it generally responds well to conservative care. However, apart from the more obvious cosmetic concerns, many parents and practitioners are becoming concerned about the developmental challenges these children may face in their future.
A case in point was a mother who reported to the chiropractor that her 8-week old infant would only look to his right hand side when placed supine. She presented photographs she had taken of her child’s head shape over the preceding weeks and explained that she was worried that the progressive flattening was escalating. From the physical examination as well as the photographic evidence, it was clear that her child was developing a unilateral flattening of the right occipital bone with associated right sided frontal bossing and facial asymmetry.
Although the cosmetic effect was discussed, being an occupational therapist herself, the mother’s concern lay chiefly with possible developmental delay associated with this condition and if the severity of the deformation related to the level of delay her child may face.
The clinical question became: “Is positional plagiocephaly associated with neurodevelopmental delay in infants and toddlers?”
Background
The term plagiocephaly derives from the Greek words ‘Plagios’ meaning oblique and ‘Kephale’ meaning head.1 The literature divides plagiocephaly into two distinct subgroups: synostotic and non-synostotic.2,3,4
Synostosis, a congenital condition involving the premature fusion of one or more cranial sutures, has an incidence of 3.5 -4.5 per 10 000 live births world-wide.5 Although the pathogenesis is not well understood, it is believed to be related to abnormalities of the osteoprogenitor cells within the cranial sutures themselves.6
Non-synostotic skull asymmetry is a subgroup in which the infant skull shape and symmetry are measurably abnormal yet their cranial sutures are apparent, normal and exhibit no early signs of fusion as in the synostotic group.7 The incidence of this group has been measured as slightly under 20% within the population, it peaks at four months and decreases to around 3.3% at two years of age.8 The hallmark of non-synostotic skull asymmetries is that they develop as a result of uneven mechanical pressures being applied to the cranial bones of the infant skull.9
Flexible sutures and malleable cranial bones are required during birth to allow the human head to navigate the birth canal and also through the first seven months of life as it is during this rapid growth period that the infant’s cerebellar volume doubles.10 However, it is this same mobility and plasticity within the structures of the infant cranium that allow for deformation if compressed, for instance, against mother’s pelvic rim or lumbosacral spine during the last months of intrauterine life.11 The cranium may also be affected by uneven pressures associated with the birthing process or positional stress in the postnatal period.7 However, asymmetries occurring during the intrauterine period, or perinatally tend to reduce spontaneously in children without impaired motor delay12 and so the diagnosis of positional plagiocephaly (PP) can only be applied from the sixth week of life.10,12
The typical presentation of infant PP includes unilateral occipital flattening with associated anterior translation of the ipsilateral ear, cheek and ipsilateral frontal bossing resulting in a parallelogram shape, with the head shifted forward on the side of occipital compression.1,13 Other presentations of skull asymmetries include brachycephaly, a bilaterally flattened occiput resulting in a short skull anterior to posterior10 and scaphocephaly, with a head shape resembling an inverted boat with a keel elongated anterior to posterior.14
PP has become the most frequent condition presenting to craniofacial clinics15 and is counted as the leading cause of skull asymmetries in infants.16 A major causative factor, has been the enforced supine sleep protocol prescribed by the Back to Sleep campaign initiated in 1992 by the American Association of Pediatricians in a relatively successful bid to curb cases of sudden infant death syndrome.15,17 An unintended consequence of the campaign has been unremitting, constant pressure on the occiputs of sleeping children leading to a six-fold increase of PP cases with almost 50% of western infants observed to have some degree of skull deformity.1
Previous authors have labelled PP as purely cosmetic,7 yet a growing body of work suggests the possibility of neurodevelopmental impact on these infants.9,11,18 There is limited information however as to the effect the severity of the PP plays in these delays.19 Evidence does exist to suggest that cortical structures can ‘shift’ in response to PP deformities9 revealing findings of a shortened corpus callosum and a greater height and height-width ratio of the cerebellar vermis seen in PP cases. Although these cortical aberrations have been observed, it is not understood if they would have any functional effects on the neurodevelopment of these infants.
Method
The electronic databases Pubmed, Medline and The Cochrane Library were searched. The two Mesh terms: ‘plagiocephaly, nonsynostotic’ and ‘growth and development’ were searched with the Boolean operator ‘AND’. The following searches cross referenced ‘plagiocephaly, nonsynostotic’ with ‘neurodevelopmental delay’, development’ and ‘delay’. Studies were included if they were conducted in English and involved human subjects only. Studies were excluded if they involved cases of synostosis, other congenital anomalies or involved otherwise ill infants. Studies conducted prior to 1992 were disregarded as those subjects would not have been under the influence of the Back to Sleep campaign. To be of sufficient quality to be included in the review, studies had to make use of a validated scale when assessing developmental delay. As there is no one validated measure of head circumference, all types of reliable measurement were included. Case studies were not included and trials had to involve more than 20 subjects. The initial database search yielded 40 articles with five others obtained through hand searches. Duplicates were removed and records were screened for their content leaving a remainder of 23. These articles had their full text assessed leading to resulting in 12 studies being included for review. See Figure 1.
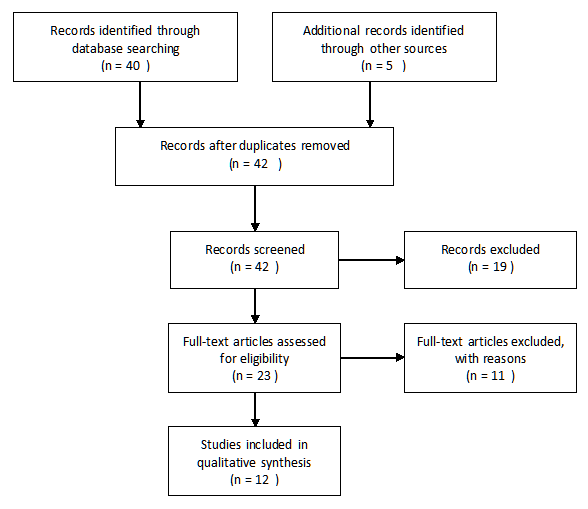
Figure 1: Flow chart of article identification process.
Results
The results are tabulated in Table 1. Regarding exposure measures, anthropometric (manual) two dimensional measurements, as used by Hussein et al.19 and Fontana et al.21 are the oldest objective measure of PP.12 They are often difficult to perform especially with fussy infants and have poor reproducibility.22 Speltz et al. and Collett et al. both used 3D imaging to diagnose PP.9,24 Although this method generates good data, it is difficult to use in day to day practice because of expense.25 However, Nahles et al. showed no discernible differences when the two aforementioned methods were compared for accuracy and both methods were well accepted.12

Table 1: Results.
The Headsup! Methodology used by Hutchison3,18,26 made use of oblique cranial length ratio, which is a recommended measure of PP.27 Knight et al. used the Argenta classification20 which has been shown to have highly reproducible and reliable results.15,28 A drawback of the Argenta classification system is that severity of the individual abnormalities is not reflected.10 Kennedy et al., Kordestani et al. and Fowler et al. explained that PP was radiographically diagnosed but did not explain which measurements were taken.2,4,29 There is no standardization for head shape measurement3 but the fact that all measures used are reliable increases validity. The heterogeneity of measurement types does however create a certain amount of limitation in terms of comparing the data.
Regarding outcomes measures, only four of the 11 included trials involved control groups. The remaining studies compared PP infant’s developmental outcomes against normative data supplied by the various developmental tests. The issue with using normative values is the possibility of demographic bias, as normative values of specific tests may not represent infants in the sample group of the particular study, leading to incorrect outcomes.23,30 Normative data may be subject to the ‘cohort effect’.
All included studies, apart from one2, noted developmental delay in children suffering PP. When looking at subgroups within developmental delay, three studies noted the motor component to be the most affected,20,23,26 with one study finding over 50% of cases to be associated with torticollis.3 One study found language development to be delayed but not cognitive function.21 Going forward it was seen that developmental delay associated with PP reduced as children approached 12 months26 and at follow up at three to four years of age, developmental delay associated with PP was seen to improve dramatically.18 Four separate studies reached the similar conclusion that the degree of developmental delay and the severity of PP were not seen to correlate.
Discussion
The goal of this study was to investigate the published literature to determine any association of positional plagiocephaly (PP) with neurodevelopmental delay. Studies predominately showed an association between the two conditions. Four of the twelve studies found none. Interestingly, four of the studies suffered from small population size. Small sample sizes are known to have reduced capacity to identify relationships between neurodevelopmental outcome and predictive factors.20 With this in mind, the data from these studies was weighed accordingly. Small sampling could be behind the contrary findings of no significant developmental difference between PP and non-PP infants.2
In terms of this review, the cohort effect relates to the effect the Back to Sleep campaign has on motor development as it is known that prone sleepers attain motor milestones faster than supine sleepers.31 Interestingly, Kennedy et al. found lack of prone awake time to be the hallmark of delay in both PP and non PP groups.2 The ASQ29, PDMS2 and BSID-24 tests all suffer from the fact that they were developed before 1992 predating the time when widespread supine sleep protocols were the norm.3 ASQ-3, AIMS and BSID-3 were developed after Back to Sleep26 meaning these studies were potentially prone to less demographic bias. In conjunction with ASQ, Fowler et al. used the HINA assessment but did not explain if the examiner was trained in this type of assessment.29 Most studies fell short in one or other aforementioned areas. However the standard of methodology across most studies was deemed to be of moderate to high level.30
Special mention must be made of torticollis, as it is often associated with PP2 and may be a major predisposing factor in PP, excluding brachycephaly.19 Torticollis is present in 20% of children with PP but only 0.1-2% of children with normal skull shape.32 Whilst it may impair motor development, however, Speltz et al. found that torticollis was not linked to neurodevelopmental delay in and of itself.23
The theoretical idea that the resulting shift in the shape of brain parenchyma in response to PP skull asymmetry affects neurodevelopmental delay, does not correlate with the findings that PP severity is not linked to level of delay in infants.19,20 Severity of PP cannot, therefore, be used as a useful indicator of level of neurodevelopmental functioning.
While there is majority consensus that PP infants are more likely to suffer developmental delay, it is not a given that the cause itself is the skull asymmetry.12 Collett et al. postulated from their data that PP may be the end result of the combination of positional practices and neurodevelopmental vulnerability.24 Their findings also included previously undiagnosed PP children mistakenly placed in their control group as also scoring lower on the BSID-3 which strengthens the link whilst minimizing possible bias. The Fowler et al. findings of abnormal muscle tone, making it more difficult for these children to reposition themselves and so more likely to develop deformations, is consistent with this premise.29
The evidence-based outlook on PP according to the available data should be to treat PP as an early marker of developmental risk that is evidenced before delay fully manifests and is testable.24,30 The Collett et al. finding that neurodevelopmental delay is still evident up to 36 months of age in PP children adds further weight to the need for early screening and prevention.24
Conclusion
The data consistently shows an association between positional plagiocephaly and neurodevelopmental delay in infants which manifests in delayed early motor skills. However, when viewed in context, the link seems to be more a correlation than a causation. It is also possible that the causative relationship is reversed in many cases where pre-existing delayed development makes infants vulnerable to PP owing to their lack of mobility. In most cases it is likely to be a combination of supine sleep, lack of prone playtime, variable tone, low activity levels, male gender and neck muscle dysfunction that results in the delays that are seen on testing in PP infants.
Acknowledgements
This work did not receive funding support. No conflict of interests are understood, by the author, to exist.
References
1. de Ribaupierre, S., Vernet, O., Rilliet, B., Cavin, B., Kalina, D. and Leyvraz, P., 2007. Posterior positional plagiocephaly treated with cranial remodeling orthosis. Swiss Med Wkly, 137 (25-26), 368-372.
2. Kennedy, E., Majnemer, A., Farmer, J. P., Barr, R. G. and Platt, R. W., 2009. Motor development of infants with positional plagiocephaly. Phys Occup Ther Pediatr, 29 (3), 222-235.
3. Hutchison, B. L., Stewart, A. W. and Mitchell, E. A., 2009. Characteristics, head shape measurements and developmental delay in 287 consecutive infants attending a plagiocephaly clinic. Acta Paediatr, 98 (9), 1494-1499.
4. Kordestani, R. K., Patel, S., Bard, D. E., Gurwitch, R. and Panchal, J., 2006. Neurodevelopmental delays in children with deformational plagiocephaly. Plast Reconstr Surg, 117 (1), 207-218; discussion 219-220.
5. Byun, I. H., Hong, J. W., Hussein, M. A. and Kim, Y. O., 2018. Demographic characteristics of craniosynostosis patients in Asia. J Craniomaxillofac Surg, 46 (4), 674-678.
6. Slater, B. J., Lenton, K. A., Kwan, M. D., Gupta, D. M., Wan, D. C. and Longaker, M. T., 2008. Cranial sutures: a brief review. Plast Reconstr Surg, 121 (4), 170e-178e.
7. Bialocerkowski, A. E., Vladusic, S. L. and Howell, S. M., 2005. Conservative interventions for positional plagiocephaly: a systematic review. Dev Med Child Neurol, 47 (8), 563-570.
8. Hutchison, B. L., Hutchison, L. A., Thompson, J. M. and Mitchell, E. A., 2004. Plagiocephaly and brachycephaly in the first two years of life: a prospective cohort study. Pediatrics, 114 (4), 970-980.
9. Collett, B. R., Aylward, E. H., Berg, J., Davidoff, C., Norden, J., Cunningham, M. L. and Speltz, M. L., 2012. Brain volume and shape in infants with deformational plagiocephaly. Childs Nerv Syst, 28 (7), 1083-1090.
10. Linz, C., Kunz, F., Böhm, H. and Schweitzer, T., 2017. Positional Skull Deformities. Dtsch Arztebl Int, 114 (31-32), 535-542.
11. Panchal, J., Amirsheybani, H., Gurwitch, R., Cook, V., Francel, P., Neas, B. and Levine, N., 2001. Neurodevelopment in children with single-suture craniosynostosis and plagiocephaly without synostosis. Plast Reconstr Surg, 108 (6), 1492-1498; discussion 1499-1500.
12. Nahles, S., Klein, M., Yacoub, A. and Neyer, J., 2018. Evaluation of positional plagiocephaly: Conventional anthropometric measurement versus laser scanning method. J Craniomaxillofac Surg, 46 (1), 11-21.
13. Skolnick, G. B., Naidoo, S. D., Patel, K. B. and Woo, A. S., 2014. Analysis of digital measures of cranial vault asymmetry for assessment of plagiocephaly. J Craniofac Surg, 25 (4), 1178-1182.
14. Kapp-Simon, K. A., Speltz, M. L., Cunningham, M. L., Patel, P. K. and Tomita, T., 2007. Neurodevelopment of children with single suture craniosynostosis: a review. Childs Nerv Syst, 23 (3), 269-281.
15. Argenta, L., David, L. and Thompson, J., 2004. Clinical classification of positional plagiocephaly. J Craniofac Surg, 15 (3), 368-372.
16. Ditthakasem, K. and Kolar, J. C., 2017. Deformational Plagiocephaly: A Review. Pediatr Nurs, 43 (2), 59-64.
17. Kane, A. A., Mitchell, L. E., Craven, K. P. and Marsh, J. L., 1996. Observations on a recent increase in plagiocephaly without synostosis. Pediatrics, 97 (6 Pt 1), 877-885.
18. Hutchison, B. L., Stewart, A. W. and Mitchell, E. A., 2011. Deformational plagiocephaly: a follow-up of head shape, parental concern and neurodevelopment at ages 3 and 4 years. Arch Dis Child, 96 (1), 85-90.
19. Hussein, M. A., Woo, T., Yun, I. S., Park, H. and Kim, Y. O., 2018. Analysis of the correlation between deformational plagiocephaly and neurodevelopmental delay. J Plast Reconstr Aesthet Surg, 71 (1), 112-117.
20. Knight, S. J., Anderson, V. A., Meara, J. G. and Da Costa, A. C., 2013. Early neurodevelopment in infants with deformational plagiocephaly. J Craniofac Surg,24 (4), 1225-1228.
21. Fontana, S. C., Daniels, D., Greaves, T., Nazir, N., Searl, J. and Andrews, B. T., 2016. Assessment of Deformational Plagiocephaly Severity and Neonatal Developmental Delay. J Craniofac Surg, 27 (8), 1934-1936.
22. Argenta, L. C., David, L. R., Wilson, J. A. and Bell, W. O., 1996. An increase in infant cranial deformity with supine sleeping position. J Craniofac Surg, 7 (1), 5-11.
23. Speltz, M. L., Collett, B. R., Stott-Miller, M., Starr, J. R., Heike, C., Wolfram-Aduan, A. M., King, D. and Cunningham, M. L., 2010. Case-control study of neurodevelopment in deformational plagiocephaly. Pediatrics, 125 (3), e537-542.
24. Collett, B. R., Gray, K. E., Starr, J. R., Heike, C. L., Cunningham, M. L. and Speltz, M. L., 2013. Development at age 36 months in children with deformational plagiocephaly. Pediatrics, 131 (1), e109-115.
25. van Lindert, E. J., Siepel, F. J., Delye, H., Ettema, A. M., Bergé, S. J., Maal, T. J. and Borstlap, W. A., 2013. Validation of cephalic index measurements in scaphocephaly. Childs Nerv Syst, 29 (6), 1007-1014.
26. Hutchison, B. L., Stewart, A. W., de Chalain, T. and Mitchell, E. A., 2012. Serial developmental assessments in infants with deformational plagiocephaly. J Paediatr Child Health, 48 (3), 274-278.
27. Aarnivala, H., Vuollo, V., Heikkinen, T., Harila, V., Holmström, L., Pirttiniemi, P. and Valkama, A. M., 2017. Accuracy of measurements used to quantify cranial asymmetry in deformational plagiocephaly. J Craniomaxillofac Surg, 45 (8), 1349-1356.
28. Branch, L. G., Kesty, K., Krebs, E., Wright, L., Leger, S. and David, L. R., 2015. Argenta clinical classification of deformational plagiocephaly. J Craniofac Surg, 26 (3), 606-610.
29. Fowler, E. A., Becker, D. B., Pilgram, T. K., Noetzel, M., Epstein, J. and Kane, A. A., 2008. Neurologic findings in infants with deformational plagiocephaly. J Child Neurol, 23 (7), 742-747.
30. Martiniuk, A. L., Vujovich-Dunn, C., Park, M., Yu, W. and Lucas, B. R., 2017. Plagiocephaly and Developmental Delay: A Systematic Review. J Dev Behav Pediatr, 38 (1), 67-78.
31. Davis, B. E., Moon, R. Y., Sachs, H. C. and Ottolini, M. C., 1998. Effects of sleep position on infant motor development. Pediatrics, 102 (5), 1135-1140.
32. Losee, J. E., Mason, A. C., Dudas, J., Hua, L. B. and Mooney, M. P., 2007. Nonsynostotic occipital plagiocephaly: factors impacting onset, treatment, and outcomes. Plast Reconstr Surg, 119 (6), 1866-1873.
|

